Abstract

Background:
Red blood cell exchange (RBCX) is an effective therapy in treatment of acute and chronic complications of sickle cell disease (SCD). It involves exchanging patient's red blood cells (RBCs) with donor RBCs to significantly lower hemoglobin S concentration without subjecting the patient to the risk of iron overload. The University of Nebraska Medical Center (UNMC) established a chronic RBCX program in November 2015, which cared for patients with multiple hemoglobinopathies. In this study, we aim to evaluate some of the outcomes of patients with SCD who joined the program.
Methods:
This is a retrospective study based on review of medical records of patients with sickle cell disease. We reviewed the health records of patients with SCD who were enrolled in the chronic RBCX program between 11/2015-8/2020 at UNMC. We included patients with SCD, regardless of age, who underwent RBCX in the outpatient setting during the study period. Data were collected to assess if RBCX influenced the frequency of SCD crisis, emergency room visits, hospitalizations, and other sickle cell-related complications.
Results:
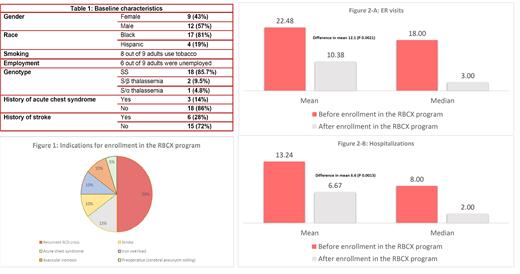
A total of 404 sessions of exchange transfusions were performed between November 2015 and August 2020 for 21 patients with SCD. The study included 9 adults (age ≥ 18 years) and 12 children with a median age of 12 years (2-31 years). During the study period, 3 adults left the program due to relocation out of state, patient's preference, or physician's decision. Table 1 summarizes the population demographic. The most common indication for enrollment in the RBCX program was recurrent sickle cell crisis (Figure 1). The mean number of emergency room visits before enrollment in the RBCX program was 22.5 visits (2-62 visits), which reduced after enrollment to 10.4 visits (0-65 visits), with a difference in mean of 12.1 visits (P=0.0021). The mean number of hospital admissions before enrollment in the RBCX program was 13.2 admissions(0-54 admissions), which also reduced to 6.7 admissions (0-50 admissions), with a significant difference in the means equal to 6. 6 admissions (P=0.0013) (Figure 2). Thirteen patients had a baseline ferritin > 500 ng/ml at enrollment; all of them had a decrease in their baseline ferritin during the study, with 4 of them achieving a new baseline < 500 ng/ml. Six patients had pre-existing antibodies at enrollment due to prior alloimmunization; however, no new alloantibodies were noticed after enrollment. The patients without preexisting antibodies were transfused with Rh and Kell matched blood. The patients with pre-existing antibodies were transfused with phenotypically matched blood. Three patients became pregnant during the study period, and their pregnancies were uncomplicated except for one patient with preeclampsia resulting in early delivery. There was no reportable death, acute chest syndrome, or stroke among the patients during the study period.
Conclusion
Outpatient chronic RBCX demonstrated safety and feasibility in both adults and children. It also showed promising outcomes in terms of reduction of sickle cell complications, number of emergency room visits and hospitalizations. These results can provide the basis for evaluating RBCX in a prospective study to better understand changes in quality of life and clinical outcomes of patients with SCD and limited therapeutic options.
Gundabolu: Pfizer: Research Funding; Samus Therapeutics: Research Funding; BioMarin Pharmaceuticals: Consultancy; Bristol-Myers Squibb Company: Consultancy; Blueprint Medicines: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal